Clinical Study Overview
Exploring the 2024 FunghiClear™ Clinical Study
Discover Key Insights
The FunghiClear™ Clinical Evaluation Study 2024
The 2024 study assessed the safety, user compliance, and clinical outcomes of FunghiClear™ Antifungal Spray. This 12-week observational trial, conducted across the Netherlands, Belgium, and the UK, included a diverse group of participants with chronic toenail fungal infections.
FunghiClear™ is the only nail spray with Manuka oil that has been scientifically demonstrated to support healthy nail regrowth. Unlike conventional fungicidal treatments, it introduces a patient-friendly approach that prioritises safety, consistency, and suitability for long-term use.
The findings suggest that FunghiClear™ is increasingly recognised by podiatrists and foot health professionals as a practical and well-tolerated option. Its results contribute to ongoing professional discussions on effective management of toenail fungus, with emphasis on safe and consistent patient care.
Study Summary: Pivotal Outcomes
The 2024 FunghiClear™ Clinical Evaluation Study followed patients across the UK, Netherlands, and Belgium over 12 weeks. Results showed the spray was well-tolerated, easy to use, and effective in supporting healthier nail growth — reinforcing its role as a practical option for podiatrists and foot health professionals.
-
Effective
31.6%
Significant enhancement in the severity rating of infected toes, from a mean of 2.53 at baseline to 3.33 at week 12 using phase cards.
-
Improvement
18.88%
Mean Onychomycosis Severity Index (OSI) score improved from 19.04 at baseline to 15.47 at week 12.
-
Easy to Use
4.2/5.0
Patients rated its convenience highly at week 6.
-
Safe to Use
99%
Users reported no pain or discomfort in the affected toenails or feet during the 12 weeks of using FunghiClear™.
Comprehensive Study Design
The 2024 FunghiClear™ Clinical Evaluation Study was conducted in the Netherlands, Belgium, and the United Kingdom. Each patient was followed for 12 weeks, with assessments carried out at three intervals during the study. This observational design applied defined inclusion and exclusion criteria to evaluate safety, reported outcomes, and ease of use. The methodology section below outlines the study phases and criteria in more detail.
-

Duration:
Each participant used FunghiClear™ Antifungal Spray twice daily for 12 weeks, with assessments at three study intervals.
-

Inclusion Criteria:
Participants were eligible
if they had PCR-confirmed dermatophyte nail infection and met age and health criteria. -

Protocol:
Treatment involved spraying affected nails daily and spraying shoes weekly to reduce reinfection risk.
-

Participants:
The study included 145 patients with an average age of 51 and mean infection duration
of 5.9 years. -

Exclusion Criteria:
Patients were excluded if using recent or ongoing antifungal treatment, or if immunocompromised.
-

Conducted By:
The study was carried out by licensed podiatrists and medical pedicurists in the Netherlands, UK, and Belgium.
Methodology
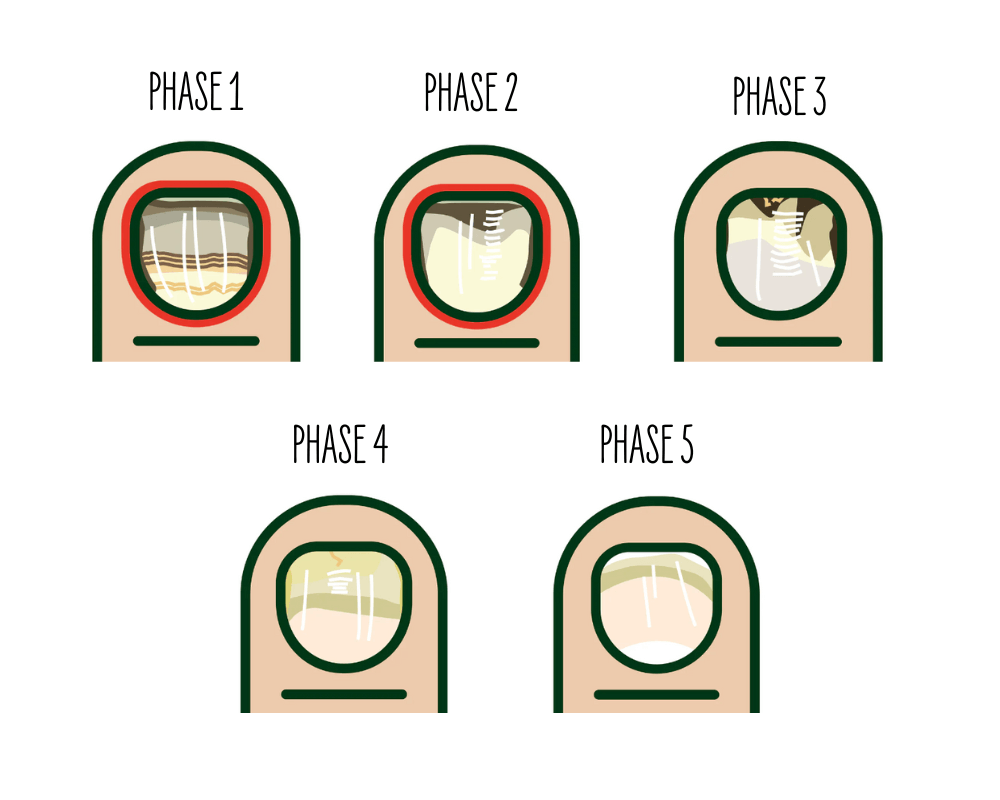

Onychomycosis Severity Index (OSI)
The OSI was used to score nail infection severity at baseline and after 12 weeks. This standard index provided a numerical measure of disease severity.

Photographic Ratings
Nails were photographed and rated at baseline, week 6, and week 12. Practitioners used phase cards to score nail condition on a five-point scale (1 = most severe, 5 = least severe), covering five clinical stages of onychomycosis. Ratings considered:
- Inflammation
- Nail structure
- Nail colour
- Signs of nail regrowth

Participant Feedback
Participants rated their levels of pain, discomfort, and convenience when using FunghiClear™ on a scale from 1 (not convenient) to 5 (very convenient). This feedback added information on ease of use and safety during the study.
Key Findings: FunghiClear™ Study 2024

Reduction in Severity
Study Result: Mean Onychomycosis Severity Index (OSI) scores decreased from 18.38 at baseline to 13.46 by week 12, representing a 26.8% improvement.
Observation: Practitioners recorded a measurable reduction in infection severity, with a 31.6% decrease in affected nail severity over the study period.

Nail Appearance Improvement
Study Result: A 22.4% improvement was recorded in nail appearance scores, from a baseline of 2.77 to 3.39 by week 12.
Observation: Ratings reflected improvements in nail structure and colour during the study period.

Safety Of Use
Participant Feedback: 99% of participants reported no pain or discomfort, with no significant adverse events noted.
Study Note: FunghiClear™ was well tolerated throughout the 12-week study.

User-Convenience
Rating: Participants reported an average convenience score of 4.4 out of 5.
Observation: Most described the spray as easy to apply within daily routines.

Mechanism of Action
FunghiClear™ contains a blend of natural oils, including Manuka, Basil, Peppermint, and Lavender. Within the study, Manuka oil was highlighted as the main active ingredient. While its antifungal mechanism is not fully established, research suggests its β-triketones may disrupt fungal cell membranes and hinder fungal growth. Laboratory data have shown Manuka oil can inhibit dermatophyte growth at concentrations below 1%.
Presentation: FunghiClear™ Study Findings and Clinical Insights
In this 30-minute presentation at the 2024 Foot and Ankle Digital Conference, Annie Salsberg, Lead Medical Director for FunghiClear™, reviews the Clinical Evaluation Study. The session outlines study design, observed outcomes, and practitioner insights, with a focus on safety, ease of use, and changes in nail condition over 12 weeks.
Concluding Remarks & Final Thoughts
The FunghiClear™ Clinical Evaluation Study 2024 reported observed improvements in nail condition, safety, and ease of use over a 12-week period. Rather than focusing only on fungal eradication, the study highlighted the importance of supporting healthy nail regrowth as part of longer-term management.
The findings suggest that FunghiClear™ may offer podiatrists and foot health professionals a practical, well-tolerated option when considering treatment approaches for patients with chronic onychomycosis.








