Clinical Study Overview
Exploring the 2024 FunghiClear™ Clinical Study
Discover Key Insights
The FunghiClear™ Clinical Evaluation Study 2024
The FunghiClear™ Clinical Evaluation Study 2024 aimed to assess the safety, effectiveness, and user convenience of FunghiClear™ Antifungal Spray. This 12-week observational study, conducted across the Netherlands, Belgium, and the UK, specifically targeted chronic toenail fungal infections, focusing on a diverse participant group.
FunghiClear™ stands out as the only product scientifically proven to support nail health with Manuka oil. Unlike traditional fungicidal treatments, which often face complications due to the fungus's resilience, FunghiClear™ introduces a significant paradigm shift by acting as a fungal inhibitor. This approach prioritises safety, compliance, and effectiveness, fostering an environment conducive to long-term, healthy nail regrowth.
The findings from this study establish FunghiClear™ as a preferred choice among healthcare professionals, advocating for a safer and more compliant alternative in the management of toenail fungus. This approach has sparked debate among podiatrists and healthcare practitioners, highlighting its practical applications and potential to revolutionise standard treatment protocols.
Study Summary: Pivotal Outcomes
Explore key findings from the FunghiClear™ Clinical Evaluation Study 2024. This summary encapsulates the safety, efficacy, and convenience of the FunghiClear™ Antifungal Spray, underscoring its significant impact on nail health management.
-
Effective
31.6%
Significant enhancement in the severity rating of infected toes, from a mean of 2.53 at baseline to 3.33 at week 12 using phase cards.
-
Improvement
18.88%
Mean Onychomycosis Severity Index (OSI) score improved from 19.04 at baseline to 15.47 at week 12.
-
Easy to Use
4.2/5.0
Patients rated its convenience highly at week 6.
-
Safe to Use
99%
Users reported no pain or discomfort in the affected toenails or feet during the 12 weeks of using FunghiClear™.
Comprehensive Study Design
The FunghiClear™ Clinical Evaluation Study 2024 was rigorously structured to assess the safety, effectiveness, and ease of use of the FunghiClear™ Antifungal Spray. Conducted in the Netherlands, Belgium, and the United Kingdom, this 12-week observational study involved a carefully selected group of participants, ensuring a thorough evaluation across diverse conditions. The following methodology section details the various phases and evaluation criteria used to determine the study's outcomes.
-

Duration:
Each participant used FunghiClear™ Antifungal Spray twice daily for a consecutive 12-week period
-

Inclusion Criteria:
Diagnosed with dermatophyte onychomycosis through:
- Positive PCR test (Dutch cohort)
- Rapid self-test (UK cohort)
-

Protocol:
Each participant followed a treatment regimen where:
- Spray was applied to toes twice daily.
- Shoes were sprayed weekly to prevent re-infection.
-

Participants:
The study included a diverse group of participants:
- Total: 145 adults
- Average Age: 51 years
- Duration of Infection: Average of 5.89 years
-

Exclusion Criteria:
Participants were excluded if they had:
- Recent antifungal treatment
- Poor wound healing due to diabetes
- Severe immunocompromised conditions
-

Conducted By:
The study was carried out by:
- Licensed podiatrists in the Netherlands, UK, and Belgium
- Medical pedicurists in the Netherlands, UK, and Belgium
Methodology
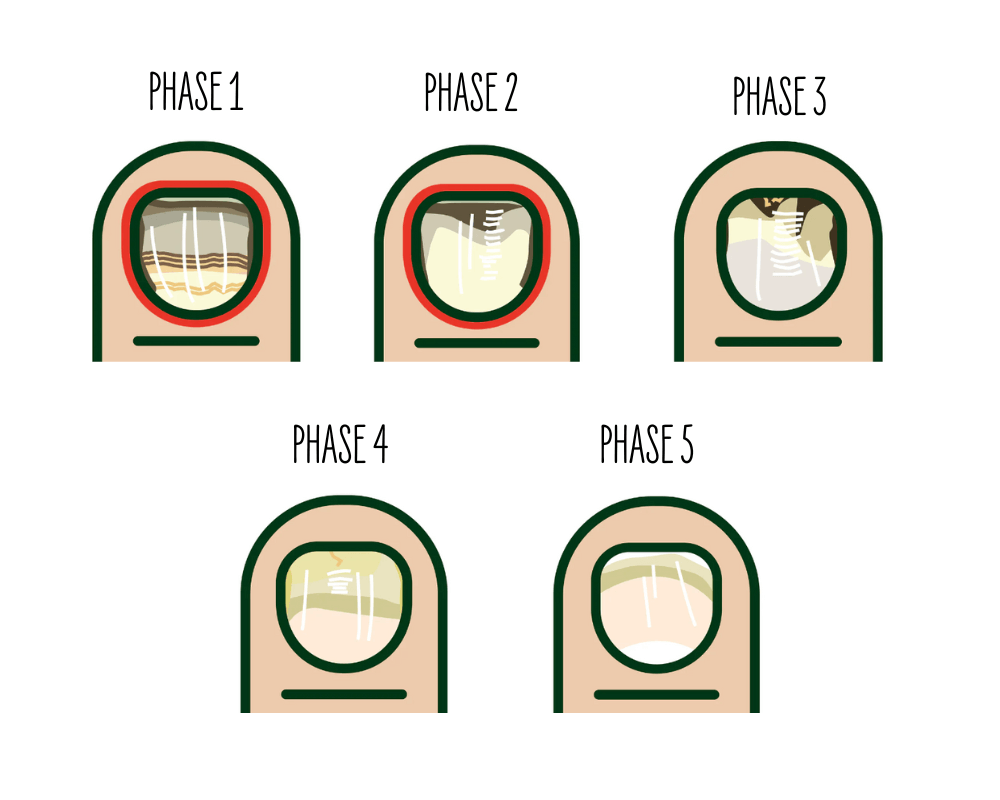

Onychomycosis Severity Index (OSI)
Used to score the severity of onychomycosis at baseline and after 12 weeks. This standard methodology provided a quantitative measure of the infection's severity.

Photographic Ratings
Nails were photographed and rated at baseline, week 6, and week 12. Practitioners used phase cards to rate the condition of the nails on a five-point severity scale (1=the worst and 5=the best), representing five clinical stages of onychomycosis. The severity assessment was based on four criteria:
- Inflammation
- Nail structure
- Nail color
- Presence of healthy nail regrowth

Participant Feedback
Participants reported their levels of pain, discomfort, and convenience using FunghiClear™ on a scale from 1 (not convenient) to 5 (very convenient). This feedback provided comprehensive data on the product's user-friendliness and safety.
Key Findings: FunghiClear™ Study 2024

Reduction in Severity
- Statistical Outcome: 26.8% improvement in mean Onychomycosis Severity Index (OSI) scores from 18.38 at baseline to 13.46 by week 12.
- Clinical Significance: Indicates a substantial reduction in the severity of toenail fungus among participants.
- Detailed Observation: Significant clinical improvement with a 31.6% reduction in infected nail severity.

Nail Appearance Improvement
- Statistical Outcome: 22.4% enhancement in the ratings of affected toenails, from a baseline of 2.77 to 3.39 by week 12.
- Visual Impact: Reflects noticeable improvements in the appearance and health of nails.
- User Feedback: High comfort score of 4.2 out of 5, indicating user satisfaction with the visible improvements.

Safety Of Use
- User Feedback: 99% of participants reported no pain or discomfort, with no significant adverse events noted.
- Safety Profile: Reinforces FunghiClear™ as a gentle and safe option for toenail fungus treatment.
- Non-Systemic Action: Minimises risks associated with systemic reactions or drug interactions, ensuring suitability for sensitive populations, including children and the immunocompromised.

User-Convenience
- User Rating: An average convenience score of 4.41 out of 5.
- Practicality: Highlights the ease and practicality of using FunghiClear™ in daily routines.
- Comfort and Satisfaction: 99% of participants enjoyed a pain-free treatment experience, emphasising the product’s user-friendly design.

Mechanism of Action
- Active Ingredients: Manuka Oil for fungal inhibition, Basil Oil for enhanced penetration, and Mint and Lavender Oils for soothing effects.
- Functional Benefits: Each ingredient plays a critical role in the overall efficacy and user experience of the treatment.
- Efficacy Observations: The unique combination of ingredients not only targets fungal infections but also supports overall nail health and regrowth.
Watch Annie Salsberg's Full Presentation on Advancing Onychomycosis Care
In this comprehensive 30-minute presentation, Annie Salsberg, the Lead Medical Director of FunghiClear™, provides an in-depth look at how FunghiClear™ offers a groundbreaking approach to managing chronic onychomycosis. Discover the science behind the product, key clinical findings, and why focusing on healthy nail regrowth, rather than solely eliminating fungal infection, can lead to better long-term outcomes for your patients.
Concluding Remarks & Final Thoughts
The FunghiClear™ Clinical Evaluation Study 2024 represents a significant advancement in toenail fungus treatment. Rather than focusing solely on the eradication of fungus, FunghiClear™ prioritises creating optimal conditions for healthy nail regrowth. This paradigm shift emphasises a balanced combination of efficacy, safety, and ease of use, acknowledging the inherent resilience of fungal infections and the crucial importance of long-term nail health.
As the only scientifically validated product utilising Manuka oil for promoting healthy nail regrowth, FunghiClear™ sets new benchmarks in podiatric care. High user satisfaction highlights its exceptional practicality, enhancing patient compliance and overall treatment success. FunghiClear™ stands out as a trusted, scientifically backed natural remedy, revolutionising toenail fungus treatment and earning the favour of healthcare professionals and patients alike








